A major part of our research is the investigation of adducts formed between nucleic acids and cytotoxic agents, such as cis-diamminedichloroplatinum(II) (cisplatin, DDP). Cisplatin is still the most widely used chemotherapeutic agent in oncology world-wide. Its cytotoxic effect is based on the formation of 1,2-intrastrand adducts at adjacent guanine nucleobases in DNA and RNA, thus, causing distortion of the double-strand and inhibiting translation. Mass spectrometric analysis of these adducts yields information on the specificity of the reagent, the stoichiometry of the reaction, and provides the armory for a rapid and unambiguous localization of adduct sites. The application of modern analytical techniques for elucidation of the modes of action of anti-cancer drugs can greatly accelerate biomedical research.
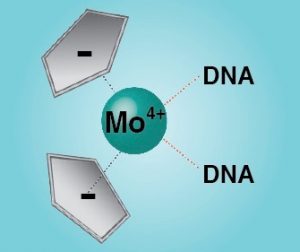
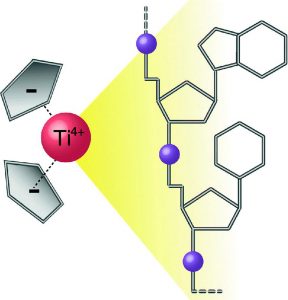
Furthermore we have extended our studies on other potential anti-cancer therapeutics. Bent metallocenes Cp2MCl2 (M = Ti, V, Nb, Mo) are known to exhibit cytotoxic activity against a variety of cancer types. Though the mechanism of action is not fully understood yet, the accumulation of the metal ions in the nucleus points towards DNA as one of the primary targets. A set of eight deoxydinucleoside monophosphates was used to study the adduct yields with metallocenes and cisplatin. The binding affinities are reflected by the relative intensities of the adducts and were found to follow the order of Pt > V > Ti > Mo (no adducts were detected with Nb). High-resolution tandem mass spectrometry was applied to locate the binding patterns in the deoxydinucleoside monophosphates. Whereas cisplatin binds to the soft nitrogen atoms in the purine nucleobases, the metallocenes additionally interact with the hard phosphate oxygen, which is in good agreement with the hard and soft (Lewis) acids and bases (HSAB) concept. However, the binding specificities were found to be unique for each metallocene. The hard Lewis acids titanium and vanadium predominantly bind to the deprotonated phosphate oxygen, whereas molybdenum, an intermediate Lewis acid, preferentially interacts with the nucleobases. Nucleobases comprise alternative binding sites for titanium and vanadium, presumably oxygen atoms for the first and nitrogen atoms for the latter. In summary, the intrinsic binding behavior of the different metallodrugs is reflected by the gas-phase dissociation of the adducts. Consequently, MS/MS can provide insights into therapeutically relevant interactions between metallodrugs and their cellular targets.
The binding of titanocene to DNA and RNA was examined by means of electrospray mass spectrometry. Titanocene served as a model for its therapeutically active derivatives. The binding preferences were probed by competition experiments with oligonucleotides of varying nucleobase compositions and sequences. Results from competition experiments revealed a generally increased preference for the binding to phosphate groups adjacent to thymidines, which is affected by the nucleobase sequence of T-rich oligonucleotides. The binding of the transition metal coordination center significantly altered the fragment ion patterns of the oligonucleotides in tandem mass spectrometric experiments. RNA was found to be less prone to adduct formation, due to intramolecular interactions. The findings from experiments on DNA and RNA were complemented by the examination of backbone- and ribose-modified oligonucleotides.
Publications
R. Eberle, S. Schürch. Titanocene Binding to Oligonucleotides. J. Inorg. Biochem., 2018, 184, 1-7. 10.1016/j.jinorgbio.2018.03.014.
R. Eberle, Y. Hari, S. Schürch. Specific Interactions of Antitumor Metallocenes with Deoxydinucleoside Monophosphates. J. Am. Soc. Mass Spectrom. 2017, 28, 1901-1909. 10.1007/s13361-017-1697-9.
Y. Hari, A. Nyakas, S. R. Stucki, S. Schürch. Elucidation of nucleic acid - drug interactions by tandem mass spectrometry. Chimia. 2014, 68, 164-167. 10.2533/chimia.2014.164
A. Nyakas, S. R. Stucki, S. Schürch. Tandem Mass Spectrometry of Modified and Platinated Oligoribonucleotides. J. Am. Soc. Mass Spectrom. 2011, 22, 875-887. 10.1007/s13361-011-0106-z
S. R. Stucki, A. Nyakas, S. Schürch. Tandem mass spectrometry of platinated quadruplex DNA. Int. J. Mass Spectrom. 2011, 46, 1288-1297. 10.1002/jms.2019
A. Nyakas, M. Eymann, S. Schürch. The Influence of Cisplatin on the Gas-Phase Dissociation of Oligonucleotides Studied by Electrospray Ionization Tandem Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2009, 20, 792-804.
